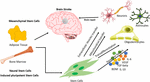
The unique capacity of stem cells of self-renewal and
differentiation has been exploited to devise cell-based therapy for
various neurodegenerative diseases, including brain stroke. There have
been several studies, which will be discussed in the upcoming
paragraphs, that report the use of stem cells in the treatment of
various diseases. These studies have used stem cells of various kinds,
such as adult stem cells (mesenchymal stem cells and neural stem cells),
embryonic stem cells, and the latest kind, induced pluripotent stem
cells. Apart from using different types of stem cells, scientists have
also reported distinctive modes of action to support their study
outcomes. Besides these variable points, there are other considerations
like the dosage of stem cells, mode of administration of stem cells, and
whether or not booster doses are required, depending upon the magnitude
of the disease. Various groups have attempted to answer these vital
questions through their research.
Ischemic stroke causes severe damage to the brain cells
by destroying the heterogeneous cell population and neuronal connections
along with vascular systems. The regenerative potential of several
types of stem cells like embryonic stem cells, neural stem cells, adult
stem cells (Mesenchymal stem cells), and induced pluripotent stem cells
have been assessed for treating stroke. The outcomes and observations in
these studies are not consistent. Most of the studies have only
commented on the homing, survival, proliferation, and differentiation of
stem cells on the site and their limited neuro-restorative ability.
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner
cell mass of the blastocyst. There have been a few studies where
engraftment of murine ESCs in mouse models of ischemia has led to the
restoration of behavioral deficits, synaptic connections, and damaged
neurons (Thomson, 1998; Wichterle et al., 2002; Nagai et al., 2010).
However, the use of ESCs in the clinical setting is argued against by
many other groups due to their immunogenic nature and teratoma-forming
tendency (Fong et al., 2010; Kawai et al., 2010; Ghosh et al., 2011).
Hence, scientists are now trying to establish the neuro-restorative
ability of other stem cell types. Neural stem cells (NSCs) are
theoretically the most appropriate cell candidates for neuro-restoration
as they belong to the same tissue source and have a natural tendency to
differentiate into neuronal cells. NSCs are multipotent cells that are
generally found in the subgranular zone of the dentate gyrus of the
hippocampus (Toda et al., 2001).
Engraftment of NSCs has been reported to lead to the reformation of
synaptic connections and improvement in the electrophysiological
properties of mature neurons in the damaged brain (Polezhaev and Alexandrova, 1984; Polezhaev et al., 1985; Cho et al., 2002; Oki et al., 2012). They do so by improving the extracellular microenvironment and hence encouraging neuronal circuit plasticity (Ourednik et al., 2002; Lee et al., 2007; Redmond et al., 2007; Jeyakumar et al., 2009).
NSCs restore neuronal functions as they secrete several neurotrophic
factors like BDNF and VEGF, which help in maintaining the health,
generation, proliferation, and survival of the neurons, along with the
maintenance of ECM (Emanueli et al., 2003; Jung et al., 2008; Lee H. J. et al., 2010; Smith et al., 2012). VEGF specifically helps in angiogenesis and vascular restoration of the blood vessels damaged due to ischemia (Song et al., 2015; Ryu et al., 2016).
CNTF, GDNF, NGF, and other such factors secreted by NSCs also play
vital roles in the protection, maintenance, and proliferation of neural
cells (Abe, 2000).
Another type of cells with amazing neuro-restorative
potential and that have several other desirable properties, like being
immunologically naive, easy to extract and maintain and expand in vitro, and not having associated ethical concerns, are mesenchymal stem cells (MSCs) (Baksh et al., 2007; Uccelli et al., 2008; Russell et al., 2018).
MSCs are multipotent stem cells that have their niche in body tissues
like bone marrow, adipose tissue, umbilical cord, umbilical cord blood,
dental pulp, etc (Uccelli et al., 2008; Singh et al., 2017; Russell et al., 2018).
Extracting MSCs from these tissues is a very well-established and easy
process and has been very widely used in various clinical trials (Nandy et al., 2014; Singh et al., 2017).
MSCs lead to neuro-restoration by one or more modes of action such as
the release of paracrine factors, cell replacement, mitochondrial
transfer, etc. MSCs also have an angiogenic effect. They have been
reported to induce angiogenesis by the release of vascular endothelial
growth factor (VEGF) (Li et al., 2000, 2001; Chen et al., 2003; Shen et al., 2007).
The only issue to be considered for using bone marrow-derived MSCs is
the surgical intervention to obtain the bone marrow. Adipose
tissue-derived MSCs have proved to be equally effective in
neuro-regeneration, with the added advantages of being easily accessible
and more abundant (Yang et al., 2012; Moore and Abrahamse, 2014; Singh et al., 2017).
Adipose tissue-derived MSCs have been known to play a protective role
through the release of extracellular vesicles. There are studies
reporting the safety and efficacy of extracellular vesicles derived from
adipose tissue-derived MSCs (Ra et al., 2011; Zhang Y. et al., 2015; Chen et al., 2016; Bang and Kim, 2019). However, more detailed studies are required to establish MSCs as therapeutic agents.
Another type of stem cell that has been explored for its
translational value recently is the induced pluripotent stem cell
(iPSC). There has been a boom in research into iPSCs after the
groundbreaking discovery by Takahashi and Yamanaka (2006).
iPSCs have the edge over other types of stem cells due to being
non-immunogenic, easy to access, and non-interventional and not giving
rise to ethical concerns. However, their generation is still an
unresolved issue, as the reprogramming efficiency is still very low.
Additionally, some studies have reported the formation of teratoma in
the mouse brain, which implies that the tumorigenicity of iPSCs needs to
be addressed and resolved before taking them into the clinical setting.
iPSCs seem to be formidable stem cells for tissue regeneration (Israel et al., 2012; Fernández-Susavila et al., 2019).
The use of complementary and alternative medicine along
with stem cell therapy also plays an important role in the recovery of
brain stroke patients. During the stroke episode, most of the
pro-inflammatory cytokines are involved, and many polyphenol compounds
extracted from different parts of medicinal plants have been shown to
protect against cerebral ischemia in pre-clinical models. Glycrrhizin
extracted from the licorice root, Glycrrhiza glabra, protected
against the rat brain injury induced by stroke. Intraperitoneal
administration of Glycrrhizin pre- and post-stroke helped inhibit the
infarction by ameliorating the IFN-γ mediated T-cell activity, which was
partially modulated by high mobility group box-1 (Xiong et al., 2016).
The use of intravenous administration of recombinant plasminogen tissue
activator (rtPA) was approved half a decade ago, but the limitations to
rtPA treatment include a narrow therapeutic window of 4.5 h post-stroke
and a high risk for hemorrhagic transformations. MSC transplantation in
brain stroke patients is an existing approach, but inflammation has
sometimes been observed in MSCs due to oxygen glucose deprivation during
treatment. One study showed that a nano-formulation of gelatin-coated
polycaprolactone loaded with naringenin, a strong anti-inflammatory,
protected the MSCs against oxygen glucose deprivation-induced
inflammation and also reduced the levels of pro-inflammatory cytokines
(TNF-α, IFN-γ, and IL-β) and of the anti-inflammatory biomarkers COX-2,
iNOS, and MPO (Ahmad et al., 2019). The active compound Eugenol, isolated from Acorus gramineus,
was tested in a cerebral ischemia perfusion rat model. Pre-treatment
with Eugenol in the rat model showed that it was prompt in attenuating
cerebral ischemic injury by inducing autophagy via the AMPK/mTOR/P70S6K
signaling pathway. In another study, the neuroprotective effect of
quercetin was demonstrated in mice, and the findings suggested that the
quercetin helped reduce apoptosis in the focal cerebral ischemia rat
brain and that the mechanism may be related to the activation of the
PI3K/Akt signaling pathway (Yao et al., 2012).
The intragastric administration of berberin and glycyrrhizin showed
neuroprotective effects in mice subjected to transient middle cerebral
artery occlusion. The co-administration of glycyrrhizin and berberin
showed more potent suppression on the HMGB1/TLR4/NF-kB pathway in
comparison to treatment with either alone. The results of the study
suggested that the administration of these compounds protects the brain
from ischemia-reperfusion injury and that the mechanism may rely on
their anti-inflammatory effects and, moreover, also by suppressing the
activation of the HMGB1/TLR4/NF-kB signaling pathway (Zhu et al., 2018).
Medicinal plants contain several important bioactive constituents that
help in several modalities. Numerous pre-clinical studies have been
performed using plant-derived products that help modulate the
proliferation and differentiation of MSCs, as well as being useful in
the field of biomaterials. Therefore, the new combination therapy of
phytochemicals along with stem cell therapy may become a new perspective
in stem cell-based neuro-regeneration.
The experimental evidence of the benefits of stem cells in treating stroke has been provided over the course of several years (Abe, 2000; Mays et al., 2010).
The usefulness of various types of stem cells has been proclaimed in
various neurological diseases, along with their safety and efficacy at
both pre-clinical and clinical levels. The pre-clinical validation of
stem cells in treating stroke has been instrumental. Various study
groups have validated the use of stem cells in terms of various
parameters such as type of stem cells, number/dose of stem cells, mode
of administration, homing and tracking of stem cells, and safety and
efficacy of stem cells (Zheng et al., 2018; Borlongan, 2019).
The most commonly used and most widely explored stem
cells in the treatment of stroke are MSCs. Among the various tissue
sources of MSCs, the most common and widely explored are bone marrow and
adipose tissue, with bone marrow being the oldest of all (Andrews et al., 2008; Xin et al., 2013; Zhang et al., 2014; Zhang Y. et al., 2015). However, neural stem cells and bone marrow-derived mononuclear stem cells have also been explored (Taguchi et al., 2004; Darsalia et al., 2007; Takahashi et al., 2008). In most of the pre-clinical studies, autologous bone marrow-derived MSCs have been used (Zhang et al., 2006; Khalili et al., 2012; Otero et al., 2012; Bao et al., 2013; Vaquero et al., 2013)
to investigate the various aspects of stem cell transplantation in
stroke. Several other studies report the use of MSCs from other tissue
sources, like adipose tissue, umbilical cord, placenta, etc (Yang et al., 2012; Zhang Q. et al., 2015; Xie et al., 2016).
MSCs are characterized for transplantation based on surface marker
profiling, which includes the presence of markers like CD29, CD44, CD73,
CD90, and CD105 and the absence of CD34/45, CD14, and HLA class II.
Other critical factors that need to be considered for pre-clinical
studies are the number/dose of cells to be administered and the mode of
administration. Transplantations of MSCs range from 1 × 106 to 8 × 106 cells and are accomplished through different modes, including intravenous, intranasal, and intra-arterial (Chen et al., 2001; Shyu et al., 2006; Zhang et al., 2006; Yang et al., 2012; Ma et al., 2016; Rodríguez-Frutos et al., 2016; Borlongan, 2019).
While there is evidence that the transplanted MSCs have homed and
differentiated into neurons, astrocytes, and oligodendrocytes upon
administration through intravenous, intranasal, and intracerebral modes,
there are doubts on the migration of MSCs in the brain by the
intravenous mode (Díez-Tejedor et al., 2014).
Also, there are mixed reports on whether the transplantation of coaxed
and naive stem cells can achieve the desired outcome in terms of
functional recovery, BBB function, increased angiogenesis and
vasculogenesis, and tissue regeneration (Laso-García et al., 2019; Turnbull et al., 2019). More detailed studies need to be done to establish a definitive stem cell therapy regime for stroke.
Cerebrovascular strokes can cause morbidity and mortality
and induce long-term disability that affects quality of life. Stroke is
associated with neuroinflammation, which plays a key role in the
pathophysiology of cerebrovascular accidents of different types. We
performed a rigorous search of a database on clinical studies with
stroke and found more than 56 clinical trials on the use of regenerative
medicine (autologous or allogeneic) for cerebrovascular stroke. Most of
them used mesenchymal stem cells, adipose tissue, bone marrow-derived
cells, and spinal cord and umbilical cord cells. Table 1
presents a few clinical trials involving stem cell therapy (autologous
and allogeneic), giving their study design, dose, route of
administration, and outcomes. Our experience with regenerative medicine
in stroke emphasizes the safety and tolerance of cells, whereas efficacy
still needs to be addressed. More recovery in clinical and functional
patterns was observed in patients administered with autologous bone
marrow-derived cells than in the group with physiotherapy alone. We also
tried to elucidate correlations between functional MRI and outcome
after stroke, with increased activation in premotor and primary motor
areas (PM and SMA), and contralesional M1 over activation. Our present
randomized controlled trial studying the paracrine effects of autologous
mononuclear stem cells in interim showed increased VEGF and BDNF
post-treatment in all stroke patients, suggesting endogenous recovery
after restorative therapies like stem cells and a structured
neuro-rehabilitation regime. To counter the progression of the cerebral
vascular disease post-stroke and repair the damage induced in different
regions of the brain, various clinical trials with different stem cells
like mesenchymal stem cells, adipose tissue-derived stem cells, and bone
marrow mononuclear stem cells are ongoing (Table 1) that investigate potential efficacy and safety, without the occurrence of any adverse or severely adverse events.
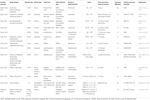
Table 1. List of Clinical trials using Stem cells in treating stroke.
An open-labeled observer-blind
clinical trial was conducted to evaluate the long-term safety and
efficacy of autologous MSCs. Post-transplantation with MSCs, clinical
improvement in patients was observed in the MSC-treated patient group,
which was associated with the serum level of stromal cell-derived
factor-1 and the degree of involvement of the sub-ventricular region of
the lateral ventricle. No serious adverse effects were observed during
long-term follow up of patients. The occurrence of comorbidities was
similar in comparison to the control group (Lee J. S. et al., 2010).
In another single-blind controlled phase I/II trial, patients with
middle cerebral artery stroke were enrolled in the study. Autologous
bone marrow mononuclear cells (BM-MNCs) were injected 5–9 days
post-stroke. A higher plasma β-nerve growth factor level was observed
post-injection, and no adverse events were observed for 6 months apart
from two patients in whom partial seizures were observed at 3 months of
follow up. The study result suggested that intra-arterial administration
of BM-MNCs is safe and feasible (Moniche et al., 2012).
A non-randomized observational controlled study with autologous bone
marrow mononuclear cells in chronic ischemic stroke showed better
efficacy and did not observe any adverse effects or neurological
abnormalities during long-term follow up of patients (Bhasin et al., 2012).
Intravenous administration of autologous BM-MSCs was also shown to have
better safety in a randomized, phase II, multicentric trial group in
patients with subacute ischemic stroke (Prasad et al., 2014).
On the basis of the findings of pre-clinical studies with peripheral
blood stem cells (PBSCs), randomized single-blind controlled studies
were conducted in patients with middle cerebral artery infarction.
Patients were enrolled as per the inclusion criteria of the study and
received subcutaneous G-CSF injection for 5 consecutive days prior to
stereotaxic implantation of immune-sorted PBSCs. No adverse events were
observed during the study procedure or the follow up of the study.
Clinical outcomes of the PBSC-treated and control groups were observed
in terms of changes in NIHSS, ESS, EMS, and mRS from baseline to 12
months. Moreover, this study also provided important evidence on the
efficacy of PBSCs in improving stroke-related motor deficits, the
reconstruction of injured CST, and the rebuilding of electrophysiology
activity from the brain to the limbs (Chen et al., 2014).
Intravenous administration of allogeneic mesenchymal stem cells from
adipose tissue in a phase II randomized, double-blind, placebo
controlled single-center pilot clinical trial in patients 2 weeks
post-acute stroke showed better efficacy without the occurrence of
adverse events. Moreover, the use of allogenic MSCs could be an
alternative therapy for the treatment of stroke because it has been
demonstrated that they lack class II HLA antigens (Díez-Tejedor et al., 2014). Another study (Bhasin et al., 2016)
reported the paracrine mechanism of bone marrow-derived mononuclear
cells in chronic ichemic stroke patients. CD34+ was counted in BM-MNCs
for each and every patient. Intravenously administered BM-MNCs secrete
glial cell-derived neurotrophic factor and BDNF, IGF-1, and VGEF, which
may protect against the dysfunction of motor neurons. The trial results
suggested that the administration of BM-MNCs is safe and feasible for
stroke patients. In another phase I, open-label, prospective clinical
trial, patients with acute ischemic stroke received a single i.v.
infusion of allogeneic human umbilical cord blood cells within a window
of 3–10 days. Post-UCB infusion, graft-vs.-host disease, infection, and
hypersensitivity were analyzed at patient follow up visits at 3, 6, and
12 months. Adverse events and severe adverse events (AE/SAE) in the
patients that were directly or indirectly related to the investigational
treatment were reported (Laskowitz et al., 2018).
A single-arm, phase I clinical trial study of autologous
bone marrow mononuclear cells for acute ischemic stroke showed a
promising new investigational modality that may help widen the
therapeutic window for patients with ischemic stroke. AEs/SAEs were
observed post-transplantation, some of which may have been associated
with the intervention but others of which may not have (Vahidy et al., 2019).
In another single-site phase I study, the feasibility and safety of
NSI-566 primary adherent neural cells derived from a single human fetal
spinal cord were observed. Three different doses were investigated in a
cohort study of patients, and it was shown that the transplantation of
human spinal cord-derived neural stem cells into the peri-infarct area
of stable stroke patients is beneficial. The mechanism potentially
behind it is that the stem cell-derived tissue is largely composed of
interneurons and glial cells, and these promote regeneration and act as
bridges between regenerating neuronal fibers (Zhang et al., 2019).
A phase I/II preliminary safety and efficacy study of allogenic MSCs in
chronic stroke patients showed the dose tolerability to be 1.5
million/kg body weight in phase I and phase II study. The primary
outcome of intravenous administration of allogenic MSCs in patients was
measured for 1 year, and secondary outcomes were measured in terms of
behavioral changes. AEs/SAEs were observed in 13 patients that were
probably not related to the intervention, and two mild AEs related to
the study intervention were observed, urinary tract infection and
intravenous site irritation. However, other mechanisms have also been
shown that involve cell replacement, immunomodulatory action, and
endogenous repair of brain damage post-stroke. The stem cell therapy in
cerebrovascular accident depends overall upon their differentiation,
inflammation, and ability to repair of endogenous processes. This
regenerative medicine has emerged as an important tool in modern
neurology, with potential efficacy in neurodegenerative disorder (Thwaites et al., 2012; Yu et al., 2013).
After extensive findings of pre-clinical research, the clinical trials
have conducted with different stem cells in stroke, in which the
AEs/SAEs observed during or post transplantation may be directly or
indirectly related to the intervention. The studies suggest that there
must be a further continuation of pre-clinical and clinical studies of
regenerative medicine in stroke patients to further elucidate the
safety, efficacy, and toxicity pre and posting transplantation and their
capacity to deliver potent efficacious regenerative medicine for stroke
patients. Further clinical trials of regenerative medicine in
cerebrovascular stroke are complete, with more results awaited.
Future Prospects
Regenerative medicine is looking increasingly more
enticing as we capture more evidence from past and current clinical
trials in stroke (Bhasin et al., 2016, 2017).
The neurophysiology describing stem cells and their concatenated
mechanisms suggests that restoration of brain function may be a
realistic goal. There are several cellular labeling techniques
available, including simple incubation, use of transfection agents,
magnetoelectroporation, and magnetosonoporation. MR tracking with SPIOs
and nanoparticles in a MCAo occlusion model of stroke has proven
flawless in tracking cells but still needs clinical validation (Cromer Berman et al., 2011).
To make this research a therapeutic boon in stroke, certain questions
still need answers, such as the optimal cell delivery route, the initial
engraftment and distribution pattern of injected cells, and how
effectively injected cells migrate toward the affected sites.
While stem cells have proven to be a great resource for
treating stroke, there are still several obstacles to be conquered in
the near future. A variety of stem cells with multiple parameters have
been under trial for the treatment of stroke. Starting from the kinds of
stem cells in use, there are pluripotent stem cells (ESCs and iPSCs),
neural stem cells, and adult stem cells (MSCs from various tissues).
There are ethical concerns associated with pluripotent stem cells.
Additionally, NSCs have limitations in their in vitro expansion
(in terms of the number of NSCs required to be transplanted). MSCs are
capable of combating this concern. Another issue is immunological
tolerance between the host body and transplanted stem cells. This issue
can be resolved by using the patient’s own cells to derive iPSCs of MSCs
(as they are devoid of HLA class II). Besides these concerns, there are
several other concerns, such as whether the efficiency of cell
extraction, expansion, and differentiation is sufficient for
transplantation, as well as the best mode of injection and optimal
number of injections. While there are several challenges to bringing
stem cell therapy in the mainstream of treatment for various diseases,
stem cell therapy has been established for treating several degenerative
and other kinds of diseases. In future, all these points of concern
need to be addressed to make stem cell therapy an abiding treatment
regime for stroke.
Author Contributions
MS, AB, and PP: drafting and refining the manuscript. SM,
MS, and AB: critical reading of the manuscript. All of the authors have
read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in
the absence of any commercial or financial relationships that could be
construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Sonali Rawat, Ph.D. scholar, Stem Cell
Facility, AIIMS, New Delhi, for helping us with the generation of the
figure and graphical abstract.
References
Abe, K. (2000). Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 20, 1393–1408. doi: 10.1097/00004647-200010000-00001
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahmad, A., Fauzia,
E., Kumar, M., Mishra, R. K., Kumar, A., Khan, M. A., et al. (2019).
Gelatin-coated polycaprolactone nanoparticle-mediated naringenin
delivery rescue human mesenchymal stem cells from oxygen glucose
deprivation-induced inflammatory stress. ACS Biomater. Sci. Eng. 5, 683–695. doi: 10.1021/acsbiomaterials.8b01081
CrossRef Full Text | Google Scholar
Andrews, E. M.,
Tsai, S. Y., Johnson, S. C., Farrer, J. R., Wagner, J. P., Kopen, G. C.,
et al. (2008). Human adult bone marrow-derived somatic cell therapy
results in functional recovery and axonal plasticity following stroke in
the rat. Exp. Neurol. 211, 588–592. doi: 10.1016/j.expneurol.2008.02.027
PubMed Abstract | CrossRef Full Text | Google Scholar
Baksh, D., Yao, R.,
and Tuan, R. S. (2007). Comparison of proliferative and multilineage
differentiation potential of human Mesenchymal stem cells derived from
umbilical cord and bone marrow. Stem Cells 25, 1384–1392. doi: 10.1634/stemcells.2006-0709
CrossRef Full Text | Google Scholar
Bang, O. Y., and
Kim, E. H. (2019). Mesenchymal stem cell-derived extracellular vesicle
therapy for stroke: challenges and progress. Front. Neurol. 10:211. doi: 10.3389/fneur.2019.00211
CrossRef Full Text | Google Scholar
Bao, X. J., Liu, F.
Y., Lu, S., Han, Q., Feng, M., Wei, J. J., et al. (2013).
Transplantation of FLK-1+ human bone marrow-derived mesenchymal stem
cells promotes behavioral recovery and anti-inflammatory and
angiogenesis effects in an intracerebral hemorrhage rat model. Int. J. Mol. Med. 31, 1087–1096. doi: 10.3892/ijmm.2013.1290
PubMed Abstract | CrossRef Full Text | Google Scholar
Bhasin, A., Kumaran,
S. S., Bhatia, R., Mohanty, S., and Srivastava, M. V. P. (2017). Safety
and feasibility of autologous mesenchymal stem cell transplantation in
chronic stroke in Indian patients. A four-year follow up. J. Stem Cells Regen. Med. 14, 59–60.
Google Scholar
Bhasin, A., Padma
Srivastava, M. V., Mohanty, S., Vivekanandhan, S., Sharma, S., Kumaran,
S., et al. (2016). Paracrine mechanisms of intravenous bone
marrow-derived mononuclear stem cells in chronic ischemic stroke. Cerebrovasc. Dis. Extra 6, 107–119. doi: 10.1159/000446404
PubMed Abstract | CrossRef Full Text | Google Scholar
Bhasin, A.,
Srivastava, M. V., Bhatia, R., Mohanty, S., Kumaran, S. S., and Bose, S.
(2012). Autologous intravenous mononuclear stem cell therapy in chronic
ischemic stroke. J. Stem Cells Regen. Med. 8, 181–189.
PubMed Abstract | Google Scholar
Bliss, T. M.,
Andres, R. H., and Steinberg, G. K. (2010). Addendum to “Optimizing the
success of cell transplantation therapy for stroke”. Neurobiol. Dis. 37, 275–283. doi: 10.1016/j.nbd.2010.03.001
CrossRef Full Text | Google Scholar
Chen, D. C., Lin,
S. Z., Fan, J. R., Lin, C. H., Lee, W., Lin, C. C., et al. (2014).
Intracerebral implantation of autologous peripheral blood stem cells in
stroke patients: a randomized phase II study. Cell Transplant 23, 1599–1612. doi: 10.3727/096368914X678562
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, J., Li, Y.,
Katakowski, M., Chen, X., Wang, L., Lu, D., et al. (2003). Intravenous
bone marrow stromal cell therapy reduces apoptosis and promotes
endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 73, 778–786. doi: 10.1002/jnr.10691
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, J., Li, Y.,
Wang, L., Zhang, Z., Lu, D., Lu, M., et al. (2001). Therapeutic benefit
of intravenous administration of bone marrow stromal cells after
cerebral ischemia in rats. Stroke 32, 1005–1011. doi: 10.1161/01.STR.32.4.1005
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, K. H., Chen,
C. H., Wallace, C. G., Yuen, C. M., Kao, G. S., Chen, Y. L., et al.
(2016). Intravenous administration of xenogenic adipose-derived
mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly
reduced brain infarct volume and preserved neurological function in rat
after acute ischemic stroke. Oncotarget 7, 74537–74556. doi: 10.18632/oncotarget.12902
PubMed Abstract | CrossRef Full Text | Google Scholar
Cho, T., Bae, J.
H., Choi, H. B., Kim, S. S., McLarnon, J. G., Suh-Kim, H., et al.
(2002). Human neural stem cells: electrophysiological properties of
voltage-gated ion channels. Neuroreport 13, 1447–1452. doi: 10.1097/00001756-200208070-00020
PubMed Abstract | CrossRef Full Text | Google Scholar
Cromer Berman, S. M., Walczak, P., and Bulte, J. W. M. (2011). Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 3, 343–355. doi: 10.1002/wnan.140
CrossRef Full Text | Google Scholar
Darsalia, V.,
Kallur, T., and Kokaia, Z. (2007). Survival, migration and neuronal
differentiation of human fetal striatal and cortical neural stem cells
grafted in stroke-damaged rat striatum. Eur. J. Neurosci. 26, 605–614. doi: 10.1111/j.1460-9568.2007.05702.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Díez-Tejedor, E.,
Gutiérrez-Fernández, M., Martínez-Sánchez, P., Rodríguez-Frutos, B.,
Ruiz-Ares, G., Lara, M. L., et al. (2014). Reparative therapy for acute
ischemic stroke with allogeneic mesenchymal stem cells from adipose
tissue: a safety assessment: a phase II randomized, double-blind,
placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 23, 2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Emanueli, C.,
Schratzberger, P., Kirchmair, R., and Madeddu, P. (2003). Paracrine
control of vascularization, and neurogenesis by neurotrophins. Br. J. Pharmacol. 140, 614–619. doi: 10.1038/sj.bjp.0705458
PubMed Abstract | CrossRef Full Text | Google Scholar
Feng, Y., Tian, G.
P., Li, L., and Zhou, J. (2014). Effect of human umbilical cord
blood-derived mesenchymal stem cells in the treatment of cerebral
infarction. Pract. J. Cardiac. Cereb. Pneumal. Vasc. Dis. 22, 28–30.
Google Scholar
Fernández-Susavila,
H., Bugallo-Casal, A., Castillo, J., and Campos, F. (2019). Adult stem
cells and induced pluripotent stem cells for stroke treatment. Front. Neurol. 10:908. doi: 10.3389/fneur.2019.00908
PubMed Abstract | CrossRef Full Text | Google Scholar
Ghosh, Z., Huang,
M., Hu, S., Wilson, K. D., Dey, D., and Wu, J. C. (2011). Dissecting the
oncogenic and tumorigenic potential of differentiated human induced
pluripotent stem cells and human embryonic stem cells. Cancer Res. 71, 5030–5039. doi: 10.1158/0008-5472.CAN-10-4402
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, Q., Cao, M.
Y., Li, R. F., Jiang, H. W., and Ge, L. T. (2013). Safety and efficacy
on the treatment of cerebral infarction with umbilical cord mesenchymal
stem cells. Med. J. Wuhan Univ. 34, 57–70.
Google Scholar
Israel, M. A.,
Yuan, S. H., Bardy, C., Reyna, S. M., Mu, Y., Herrera, C., et al.
(2012). Probing sporadic and familial Alzheimer’s disease using induced
pluripotent stem cells. Nature 482, 216–220. doi: 10.1038/nature10821
CrossRef Full Text | Google Scholar
Jeyakumar, M.,
Lee, J. P., Sibson, N. R., Lowe, J. P., Stuckey, D. J., Tester, K., et
al. (2009). Neural stem cell transplantation benefits a monogenic
neurometabolic disorder during the symptomatic phase of disease. Stem Cells 27, 2362–2370. doi: 10.1002/stem.163
PubMed Abstract | CrossRef Full Text | Google Scholar
Jung, Y. L., Sang,
I. P., Ji, H. O., Seong, M. K., Chang, H. J., Jin, A. J., et al.
(2008). Brain-derived neurotrophic factor stimulates the neural
differentiation of human umbilical cord blood-derived mesenchymal stem
cells and survival of differentiated cells through MAPK/ERK and
PI3K/Akt-dependent signaling pathways. J. Neurosci. Res. 86, 2168–2178. doi: 10.1002/jnr.21669
PubMed Abstract | CrossRef Full Text | Google Scholar
Kawai, H.,
Yamashita, T., Ohta, Y., Deguchi, K., Nagotani, S., Zhang, X., et al.
(2010). Tridermal tumorigenesis of induced pluripotent stem cells
transplanted in ischemic brain. J. Cereb. Blood Flow Metab. 30, 1487–1493. doi: 10.1038/jcbfm.2010.32
PubMed Abstract | CrossRef Full Text | Google Scholar
Khalili, M. A.,
Anvari, M., Hekmati-Moghadam, S. H., Sadeghian-Nodoushan, F., Fesahat,
F., and Miresmaeili, S. M. (2012). Therapeutic benefit of intravenous
transplantation of mesenchymal stem cells after experimental
subarachnoid hemorrhage in rats. J. Stroke Cerebrovasc. Dis. 21, 445–451. doi: 10.1016/j.jstrokecerebrovasdis.2010.10.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Laskowitz, D. T.,
Bennett, E. R., Durham, R. J., Volpi, J. J., Wiese, J. R., Frankel, M.,
et al. (2018). Allogeneic umbilical cord blood infusion for adults with
ischemic stroke: clinical outcomes from a phase 1 SAFETY STUDY. Stem Cells Transl. Med. 7, 521–529. doi: 10.1002/sctm.18-0008
PubMed Abstract | CrossRef Full Text | Google Scholar
Laso-García, F.,
Diekhorst, L., Gómez-De Frutos, M. C., Otero-Ortega, L., Fuentes, B.,
Ruiz-Ares, G., et al. (2019). Cell-based therapies for stroke: promising
solution or dead end? Mesenchymal stem cells and comorbidities in
preclinical stroke research. Front. Neurol. 10:332. doi: 10.3389/fneur.2019.00332
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, H. J., Lim,
I. J., Lee, M. C., and Kim, S. U. (2010). Human neural stem cells
genetically modified to overexpress brain-derived neurotrophic factor
promote functional recovery and neuroprotection in a mouse stroke model.
J. Neurosci. Res. 88, 3282–3294. doi: 10.1002/jnr.22474
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, J. S., Hong,
J. M., Moon, G. J., Lee, P. H., Ahn, Y. H., Bang, O. Y., et al. (2010). A
long-term follow-up study of intravenous autologous mesenchymal stem
cell transplantation in patients with ischemic stroke. Stem Cells 28, 1099–1106. doi: 10.1002/stem.430
CrossRef Full Text | Google Scholar
Lee, J. P.,
Jeyakumar, M., Gonzalez, R., Takahashi, H., Lee, P. J., Baek, R. C., et
al. (2007). Stem cells act through multiple mechanisms to benefit mice
with neurodegenerative metabolic disease. Nat. Med. 13, 439–447. doi: 10.1038/nm1548
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y., Chen, J.,
Wang, L., Lu, M., and Chopp, M. (2001). Treatment of stroke in rat with
intracarotid administration of marrow stromal cells. Neurology 56, 1666–1672. doi: 10.1212/WNL.56.12.1666
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y., Chopp, M.,
Chen, J., Wang, L., Gautam, S. C., Xu, Y. X., et al. (2000).
Intrastriatal transplantation of bone marrow nonhematopoietic cells
improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow Metab. 20, 1311–1319. doi: 10.1097/00004647-200009000-00006
PubMed Abstract | CrossRef Full Text | Google Scholar
Ma, F. W., Deng,
Q. F., Zhou, X., Gong, X. J., Zhao, Y., Chen, H. G., et al. (2016). The
tissue distribution and urinary excretion study of gallic acid and
protocatechuic acid after oral administration of Polygonum capitatum
extract in rats. Molecules 21:399. doi: 10.3390/molecules21040399
PubMed Abstract | CrossRef Full Text | Google Scholar
Mays, R. W.,
Borlongan, C. V., Yasuhara, T., Hara, K., Maki, M., Carroll, J. E., et
al. (2010). Development of an allogeneic adherent stem cell therapy for
treatment of ischemic stroke∗. J. Exp. Stroke Transl. Med. 3, 34–46. doi: 10.6030/1939-067X-3.1.34
CrossRef Full Text | Google Scholar
Levy, M. L.,
Crawford, J. R., Dib, N., Verkh, L., Tankovich, N., and Cramer, S. C.
(2019). Phase I/II study of safety and preliminary efficacy of
intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke 50, 2835–2841. doi: 10.1161/STROKEAHA.119.026318
CrossRef Full Text | Google Scholar
Moniche, F.,
Gonzalez, A., Gonzalez-Marcos, J. R., Carmona, M., Piñero, P., Espigado,
I., et al. (2012). Intra-arterial bone marrow mononuclear cells in
ischemic stroke: a pilot clinical trial. Stroke 43, 2242–2244. doi: 10.1161/STROKEAHA.112.659409
PubMed Abstract | CrossRef Full Text | Google Scholar
Nagai, N., Kawao,
N., Okada, K., Okumoto, K., Teramura, T., Ueshima, S., et al. (2010).
Systemic transplantation of embryonic stem cells accelerates brain
lesion decrease and angiogenesis. Neuroreport 21, 575–579. doi: 10.1097/WNR.0b013e32833a7d2c
PubMed Abstract | CrossRef Full Text | Google Scholar
Nandy, S. B.,
Mohanty, S., Singh, M., Behari, M., and Airan, B. (2014). Fibroblast
Growth Factor-2 alone as an efficient inducer for differentiation of
human bone marrow mesenchymal stem cells into dopaminergic neurons. J. Biomed. Sci. 21:83. doi: 10.1186/s12929-014-0083-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Oki, K.,
Tatarishvili, J., Wood, J., Koch, P., Wattananit, S., Mine, Y., et al.
(2012). Human-induced pluripotent stem cells form functional neurons and
improve recovery after grafting in stroke-damaged brain. Stem Cells 30, 1120–1133. doi: 10.1002/stem.1104
PubMed Abstract | CrossRef Full Text | Google Scholar
Otero, L., Zurita,
M., Bonilla, C., Aguayo, C., Rico, M. A., Rodríguez, A., et al. (2012).
Allogeneic bone marrow stromal cell transplantation after cerebral
hemorrhage achieves cell transdifferentiation and modulates endogenous
neurogenesis. Cytotherapy 14, 34–44. doi: 10.3109/14653249.2011.608349
PubMed Abstract | CrossRef Full Text | Google Scholar
Ourednik, J.,
Ourednik, V., Lynch, W. P., Schachner, M., and Snyder, E. Y. (2002).
Neural stem cells display an inherent mechanism for rescuing
dysfunctional neurons. Nat. Biotechnol. 20, 1103–1110. doi: 10.1038/nbt750
PubMed Abstract | CrossRef Full Text | Google Scholar
Polezhaev, L. V.,
and Alexandrova, M. A. (1984). Transplantation of embryonic brain tissue
into the brain of adult rats after hypoxic hypoxia. J. Hirnforsch. 25, 99–106.
PubMed Abstract | Google Scholar
Polezhaev, L. V.,
Alexandrova, M. A., Vitvitsky, V. N., Girman, S. V., and Golovina, I. L.
(1985). Morphological, biochemical and physiological changes in brain
nervous tissue of adult intact and hypoxia-subjected rats after
transplantation of embryonic nervous tissue. J. Hirnforsch 26, 281–289.
PubMed Abstract | Google Scholar
Prasad, K.,
Sharma, A., Garg, A., Mohanty, S., Bhatnagar, S., Johri, S., et al.
(2014). Intravenous autologous bone marrow mononuclear stem cell therapy
for ischemic stroke: a multicentric, randomized trial. Stroke 45, 3618–3624. doi: 10.1161/STROKEAHA.114.007028
PubMed Abstract | CrossRef Full Text | Google Scholar
Ra, J. C., Shin,
I. S., Kim, S. H., Kang, S. K., Kang, B. C., Lee, H. Y., et al. (2011).
Safety of intravenous infusion of human adipose tissue-derived
mesenchymal stem cells in animals and humans. Stem Cells Dev. 20, 1297–1308. doi: 10.1089/scd.2010.0466
PubMed Abstract | CrossRef Full Text | Google Scholar
Redmond, D. E.,
Bjugstad, K. B., Teng, Y. D., Ourednik, V., Ourednik, J., Wakeman, D.
R., et al. (2007). Behavioral improvement in a primate Parkinson’s model
is associated with multiple homeostatic effects of human neural stem
cells. Proc. Natl. Acad. Sci. U.S.A. 104, 12175–12180. doi: 10.1073/pnas.0704091104
CrossRef Full Text | Google Scholar
Rodríguez-Frutos,
B., Otero-Ortega, L., Gutiérrez-Fernández, M., Fuentes, B.,
Ramos-Cejudo, J., and Díez-Tejedor, E. (2016). Stem cell therapy and
administration routes after stroke. Transl. Stroke Res. 7, 378–387. doi: 10.1007/s12975-016-0482-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Russell, A. L.,
Lefavor, R., Durand, N., Glover, L., and Zubair, A. C. (2018). Modifiers
of mesenchymal stem cell quantity and quality. Transfusion 58, 1434–1440. doi: 10.1111/trf.14597
PubMed Abstract | CrossRef Full Text | Google Scholar
Ryu, S., Lee, S.
H., Kim, S. U., and Yoon, B. W. (2016). Human neural stem cells promote
proliferation of endogenous neural stem cells and enhance angiogenesis
in ischemic rat brain. Neural. Regen. Res. 11, 298–304. doi: 10.4103/1673-5374.177739
PubMed Abstract | CrossRef Full Text | Google Scholar
Shen, L. H., Li,
Y., Chen, J., Zacharek, A., Gao, Q., Kapke, A., et al. (2007).
Therapeutic benefit of bone marrow stromal cells administered 1 month
after stroke. J. Cereb. Blood Flow Metab. 27, 6–13. doi: 10.1038/sj.jcbfm.9600311
PubMed Abstract | CrossRef Full Text | Google Scholar
Shyu, W. C., Lin,
S. Z., Chiang, M. F., Su, C. Y., and Li, H. (2006). Intracerebral
peripheral blood stem cell (CD34+) implantation induces neuroplasticity
by enhancing β1 integrin-mediated angiogenesis in chronic stroke rats. J. Neurosci. 26, 3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006
CrossRef Full Text | Google Scholar
Singh, M., Kakkar,
A., Sharma, R., Kharbanda, O. P., Monga, N., Kumar, M., et al. (2017).
Synergistic Effect of BDNF and FGF2 in Efficient Generation of
Functional Dopaminergic Neurons from human Mesenchymal Stem Cells. Sci. Rep. 7:10378. doi: 10.1038/s41598-017-11028-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Smith, E. J.,
Stroemer, R. P., Gorenkova, N., Nakajima, M., Crum, W. R., Tang, E., et
al. (2012). Implantation site and lesion topology determine efficacy of a
human neural stem cell line in a rat model of chronic stroke. Stem Cells 30, 785–796. doi: 10.1002/stem.1024
PubMed Abstract | CrossRef Full Text | Google Scholar
Song, M., Kim, Y.
J., Kim, Y. H., Roh, J., Kim, E. C., Lee, H. J., et al. (2015).
Long-term effects of magnetically targeted ferumoxide-labeled human
neural stem cells in focal cerebral ischemia. Cell Transplant 24, 183–190. doi: 10.3727/096368913X675755
PubMed Abstract | CrossRef Full Text | Google Scholar
Tae-Hoon, L., and Yoon-Seok, L. (2012). Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cir. Bras. 27, 333–339. doi: 10.1590/s0102-86502012000400009
PubMed Abstract | CrossRef Full Text | Google Scholar
Taguchi, A., Soma,
T., Tanaka, H., Kanda, T., Nishimura, H., Yoshikawa, H., et al. (2004).
Administration of CD34+ cells after stroke enhances neurogenesis via
angiogenesis in a mouse model. J. Clin. Invest. 114, 330–338. doi: 10.1172/JCI200420622
PubMed Abstract | CrossRef Full Text | Google Scholar
Takahashi, K., and
Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse
Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Takahashi, K.,
Yasuhara, T., Shingo, T., Muraoka, K., Kameda, M., Takeuchi, A., et al.
(2008). Embryonic neural stem cells transplanted in middle cerebral
artery occlusion model of rats demonstrated potent therapeutic effects,
compared to adult neural stem cells. Brain Res. 1234, 172–182. doi: 10.1016/j.brainres.2008.07.086
PubMed Abstract | CrossRef Full Text | Google Scholar
Thwaites, J. W.,
Reebye, V., Mintz, P., Levicar, N., and Habib, N. (2012). Cellular
replacement and regenerative medicine therapies in ischemic stroke. Regen. Med. 7, 387–395. doi: 10.2217/rme.12.2
PubMed Abstract | CrossRef Full Text | Google Scholar
Toda, H.,
Takahashi, J., Iwakami, N., Kimura, T., Hoki, S., Mozumi-Kitamura, K.,
et al. (2001). Grafting neural stem cells improved the impaired spatial
recognition in ischemic rats. Neurosci. Lett. 316, 9–12. doi: 10.1016/S0304-3940(01)02331-X
PubMed Abstract | CrossRef Full Text | Google Scholar
Turnbull, M. T.,
Zubair, A. C., Meschia, J. F., and Freeman, W. D. (2019). Mesenchymal
stem cells for hemorrhagic stroke: status of preclinical and clinical
research. NPJ Regen. Med. 4:10. doi: 10.1038/s41536-019-0073-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Vahidy, F. S.,
Haque, M. E., Rahbar, M. H., Zhu, H., Rowan, P., Aisiku, I. P., et al.
(2019). Intravenous bone marrow mononuclear cells for acute ischemic
stroke: safety, feasibility, and effect size from a phase i clinical
trial. Stem Cells 37, 1481–1491. doi: 10.1002/stem.3080
PubMed Abstract | CrossRef Full Text | Google Scholar
Vaquero, J.,
Otero, L., Bonilla, C., Aguayo, C., Rico, M. A., Rodriguez, A., et al.
(2013). Cell therapy with bone marrow stromal cells after intracerebral
hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy 15, 33–43. doi: 10.1016/j.jcyt.2012.10.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Wichterle, H.,
Lieberam, I., Porter, J. A., and Jessell, T. M. (2002). Directed
differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. doi: 10.1016/S0092-8674(02)00835-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Xie, J., Wang, B.,
Wang, L., Dong, F., Bai, G., and Liu, Y. (2016). Intracerebral and
intravenous transplantation represents a favorable approach for
application of human umbilical cord mesenchymal stromal cells in
intracerebral hemorrhage rats. Med. Sci. Monit. 22, 3552–3561. doi: 10.12659/MSM.900512
PubMed Abstract | CrossRef Full Text | Google Scholar
Xin, H., Li, Y.,
Cui, Y., Yang, J. J., Zhang, Z. G., and Chopp, M. (2013). Systemic
administration of exosomes released from mesenchymal stromal cells
promote functional recovery and neurovascular plasticity after stroke in
rats. J. Cereb. Blood Flow Metab. 33, 1711–1715. doi: 10.1038/jcbfm.2013.152
PubMed Abstract | CrossRef Full Text | Google Scholar
Xiong, X., Gu, L.,
Wang, Y., Luo, Y., Zhang, H., Lee, J., et al. (2016). Glycyrrhizin
protects against focal cerebral ischemia via inhibition of T cell
activity and HMGB1-mediated mechanisms. J. Neuroinflam. 13:241. doi: 10.1186/s12974-016-0705-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Yang, K. L., Lee,
J. T., Pang, C. Y., Lee, T. Y., Chen, S. P., Liew, H. K., et al. (2012).
Human adipose-derived stem cells for the treatment of intracerebral
hemorrhage in rats via femoral intravenous injection. Cell Mol. Biol. Lett. 17, 376–392. doi: 10.2478/s11658-012-0016-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Yao, R. Q., Qi, D.
S., Yu, H. L., Liu, J., Yang, L. H., and Wu, X. X. (2012). Quercetin
attenuates cell apoptosis in focal cerebral ischemia rat brain via
activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 37, 2777–2786. doi: 10.1007/s11064-012-0871-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, B., Yin,
Y., Lai, R. C., Tan, S. S., Choo, A. B. H., and Lim, S. K. (2014).
Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 23, 1233–1244. doi: 10.1089/scd.2013.0479
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, G., Li, Y.,
Reuss, J. L., Liu, N., Wu, C., Li, J., et al. (2019). Stable
intracerebral transplantation of neural stem cells for the treatment of
paralysis due to ischemic stroke. Stem Cells Transl. Med. 8, 999–1007. doi: 10.1002/sctm.18-0220
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, H., Huang,
Z., Xu, Y., and Zhang, S. (2006). Differentiation and neurological
benefit of the mesenchymal stem cells transplanted into the rat brain
following intracerebral hemorrhage. Neurol. Res. 28, 104–112. doi: 10.1179/016164106X91960
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, Q., Shang,
X., Hao, M., Zheng, M., Li, Y., Liang, Z., et al. (2015). Effects of
human umbilical cord mesenchymal stem cell transplantation combined with
minimally invasive hematoma aspiration on intracerebral hemorrhage in
rats. Am. J. Transl. Res. 7, 2176–2186.
PubMed Abstract | Google Scholar
Zhang, Y., Chopp,
M., Meng, Y., Katakowski, M., Xin, H., Mahmood, A., et al. (2015).
Effect of exosomes derived from multipluripotent mesenchymal stromal
cells on functional recovery and neurovascular plasticity in rats after
traumatic brain injury. J. Neurosurg. 122, 856–867. doi: 10.3171/2014.11.JNS14770
PubMed Abstract | CrossRef Full Text | Google Scholar
Zheng, H., Zhang,
B., Chhatbar, P. Y., Dong, Y., Alawieh, A., Lowe, F., et al. (2018).
Mesenchymal stem cell therapy in stroke: a systematic review of
literature in pre-clinical and clinical research. Cell Transplant 27, 1723–1730. doi: 10.1177/0963689718806846
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhu, J. R., Lu, H.
D., Guo, C., Fang, W. R., Zhao, H. D., Zhou, J. S., et al. (2018).
Berberine attenuates ischemia–reperfusion injury through inhibiting
HMGB1 release and NF-κB nuclear translocation. Acta Pharmacol. Sin. 39, 1706–1715. doi: 10.1038/s41401-018-0160-1
CrossRef Full Text | Google Scholar
Keywords: stroke, stem cells, mesenchymal stem cells, clinical trials, pre-clinical studies
Citation: Singh M, Pandey PK, Bhasin A, Padma MV and Mohanty S (2020) Application of Stem Cells in Stroke: A Multifactorial Approach. Front. Neurosci. 14:473. doi: 10.3389/fnins.2020.00473
Received: 04 February 2020; Accepted: 16 April 2020;
Published: 09 June 2020.
Copyright © 2020 Singh, Pandey, Bhasin, Padma and Mohanty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
The use, distribution or reproduction in other forums is permitted,
provided the original author(s) and the copyright owner(s) are credited
and that the original publication in this journal is cited, in
accordance with accepted academic practice. No use, distribution or
reproduction is permitted which does not comply with these terms.
*Correspondence: Sujata Mohanty, drmohantysujata@gmail.com
Source link
Related:
Nine Things To Know About Stem Cell Treatments – A Closer ...
https://www.closerlookatstemcells.org/stem-cells-medicine/nine-things-to-know-about-stem-cell-treatments/
FDA Warns About Stem Cell Therapies | FDA
Safety Concerns for Unproven Stem Cell Treatments
All medical treatments have benefits and risks. But unproven stem cell therapies can be particularly unsafe.
https://youtu.be/onnlZeQlai0
Related posts:


Stroke kills more women than men each year but there are preventive steps you can take to minimise your risks.





















 The calves are prone to tightening and cramping, especially after a workout, so be sure to stretch them out.
The calves are prone to tightening and cramping, especially after a workout, so be sure to stretch them out.